thermodynamics - Confused why delta ∆Q and dQ don't make sense for heat Q - Chemistry Stack Exchange

In my chemistry teacher's notes, some notations concerning the heat $Q$ are marked as inappropriate. $Q$: yes d$Q$: no $\delta Q$: yes $\Delta Q$: no In the second bullet in the screenshot below
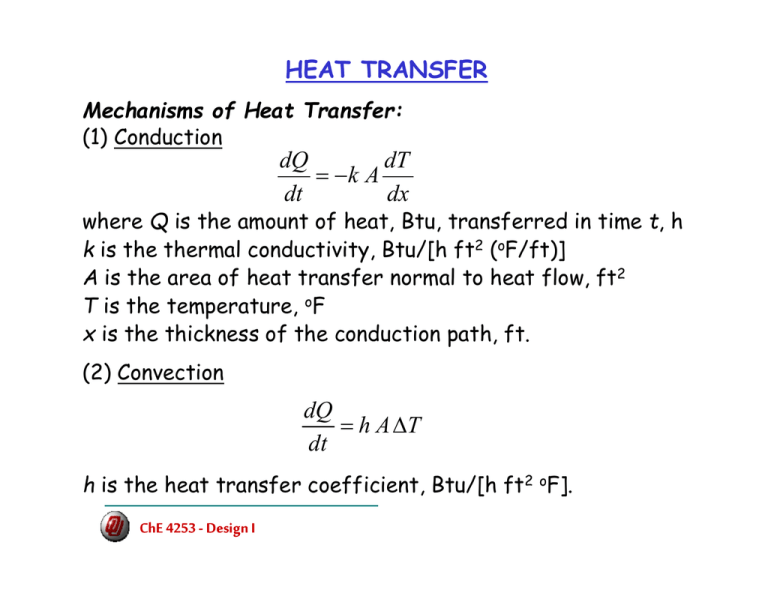
HEAT TRANSFER dx dT Ak dt dQ − = TAh dt dQ Δ =
In Heat Transfer (exchangers) is Q different to q? They seem inter-changeable. - Quora

PDF) Fundamentals of Chemical Engineering Thermodynamics
What is the relationship between the laws of thermodynamics and the nature of living systems? - Quora

First Law of Thermodynamics

Full article: Theory of cross phenomena and their coefficients beyond Onsager theorem

Putting It Into Reverse – Watts Up With That?

What relationship between scientific and traditional systems of knowledge? Some introductory remarks
What is a thermodynamic equation? - Quora
What is the basic difference between the heat of formation and the heat of reaction? - Quora

Full article: Theory of cross phenomena and their coefficients beyond Onsager theorem

Exergy - Wikipedia

In which ways are the central limit theorem and the second law of thermodynamics related? - Quora








