Medsafe blocks sales of Covid-19 'treatment' Artemisia annua

Medsafe has won praise for moving quickly to stop sales of a herbal remedy linked to Covid-19.

COVID-19 Lockdown Exit Analysis

COVID-19 in ocrelizumab-treated people with multiple sclerosis - Multiple Sclerosis and Related Disorders

Artemisia annua, possible treatment against Covid-19 : Status report o – Siyah Organics

Full article: Real-world cost of treatment for multiple sclerosis patients initiating and receiving infused disease-modifying therapies per recommended label in the United States

COVID-19: Tests for herb Artemisia begin – DW – 05/15/2020

The effect of Artemisia annua hot water extracts against the SARS-CoV-2 Omicron variant

Another Malaria Cure Draws Notice in Coronavirus Outbreak, This Time in Africa - WSJ

Artemisinin (Sweet Wormwood Cleanse)(Artemisia Annua) 200mg Per Serving, 120 Capsules (Two Month Supply) Vegan Safe, Non-GMO, Gluten Free, Manufactured in The USA (Immune Support) by Double Wood : Health & Household

Muscular Dystrophy Association Celebrates FDA Approval of Skyclarys, First Ever Treatment for Friedreich's Ataxia from Reata Pharmaceuticals

Challenges in Sedation Management in Critically Ill Patients with COVID-19: a Brief Review
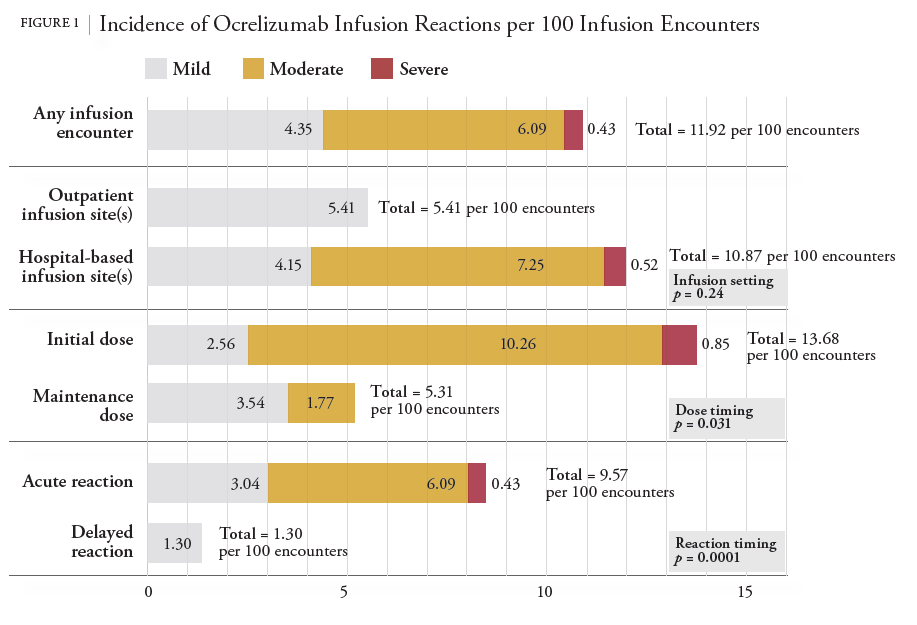
A Pilot Study of Ocrelizumab Infusion Reaction Rates Among Multiple Sclerosis Patients Treated in a Hospital and 2 Outpatient Sites of Care - National Home Infusion Association

COVID-19 Lockdown Exit Analysis

Covid 19 coronavirus: Medsafe blocks sales of 'treatment' Artemisia annua - NZ Herald

Coronavirus: What do we know about the artemisia plant?

Artemisia and Artemisia-based products for COVID-19 management: current state and future perspective