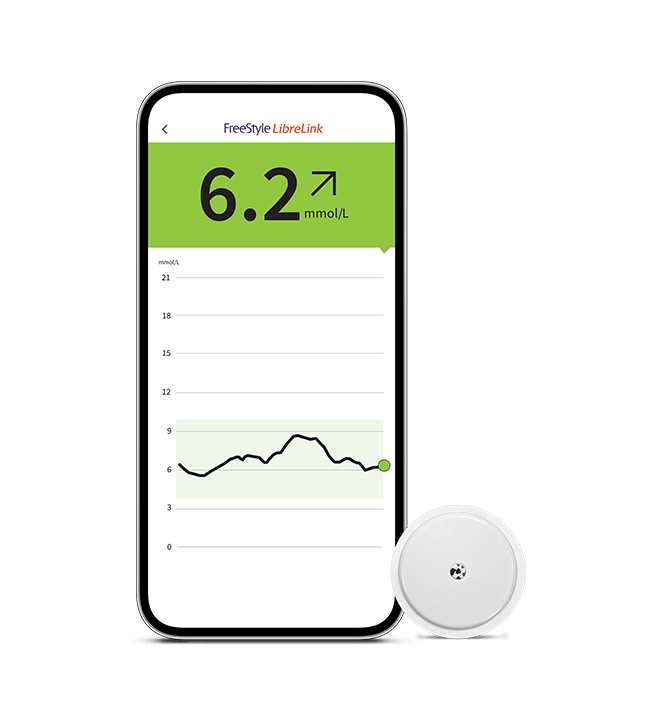
Insulet Corporation - Insulet Announces CE Mark Approval of Omnipod® 5 Integration with Abbott FreeStyle Libre 2 Plus Sensor

Omnipod 5 is the world’s first tubeless automated insulin delivery system to achieve CE mark approval with multiple continuous glucose monitoring (CGM) sensor brands. Latest Omnipod 5 integration is expected to be available in the United Kingdom and Netherlands in the first half of 2024, with additional markets to follow. Insulet Corporation (NASDAQ: PODD) (Insulet or the Company), the global leader in tubeless insulin pump technology with its Omnipod ® brand of products, today announced it has
CGM BioWorld

Diabetes Technology Updates for November 2023

Insulet Announces CE Mark Approval of Omnipod® 5 Integration with Abbott FreeStyle Libre 2 Plus Sensor - DigiBete

Insulet's Omnipod 5 Automated Insulin Delivery System, US
Insulin Delivery Device Technology — Today and Into the Future - Today's Dietitian Magazine

Medical Device Network

Insulet corp.
Emerging Diabetes Technologies: Continuous Glucose Monitors/Artificial Pancreases

Insulet Announces CE Mark Approval of Omnipod® 5 Integration with Abbott FreeStyle Libre 2 Plus Sensor

Insulet Corporation on LinkedIn: #teaminsulet

Libre Life Diabetes News February 2024, by Samantha, Love My Libre, Feb, 2024

DigiBete

Update Feb 2023ㅤ

Diabetes Care Devices Archives - Medical Device Network

PDC Health Hub by Perth Diabetes Care on LinkedIn: Insulet Announces CE Mark Approval of Omnipod® 5 Integration with Abbott…